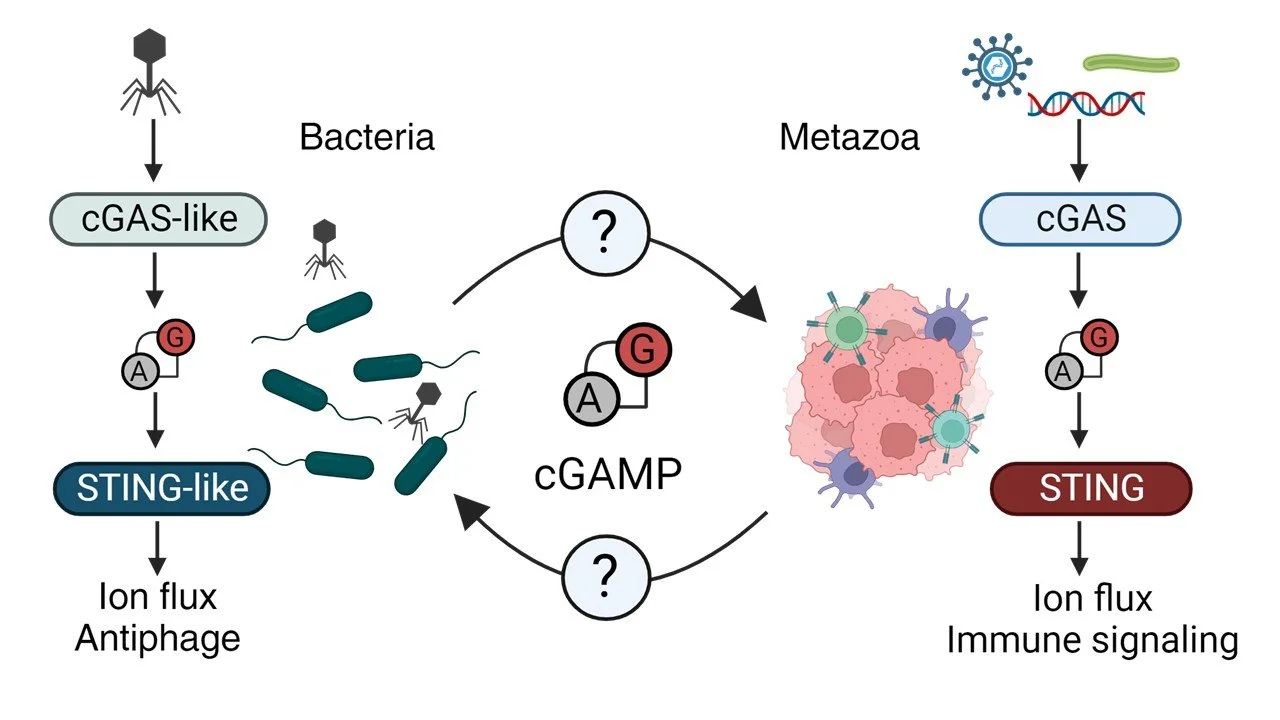
Our lab explores how bacteria sense and defend themselves against the viruses that infect and kill them: the bacteriophages (phages). To protect against viral predation bacteria evolved a vast arsenal of sophisticated immune systems that sense phage infection and execute an immune response to restrict viral replication. Remarkably, many of these bacterial antiphage systems are evolutionary progenitors to antiviral signaling pathways in eukaryotes such as the cGAS-STING pathway, which is crucial for initiating immunity to infection, cancer, and autoimmunity in humans. By studying these progenitor antiviral pathways in bacteria, we aim to identify conserved mechanistic paradigms of viral sensing and innate immune effector function that are conserved across the tree of life. We use several synergistic approaches to study these phenomena, including classical bacteriology, molecular genetics, phage virology, protein biochemistry, enzymology, electrophysiology, structural biology (cryo-EM), fluorescence microscopy, and protein engineering. Our current projects focus on the characterization of novel cyclic GMP-AMP gated channels in bacterial cGAS-like immunity to phages, discovery of novel membrane-associated signaling systems in bacterial physiology and antiviral immunity, discovery of novel bacteriophages and their biology, and engineering bacterial immune components to create programmable nucleotide-activated biotechnology for synthetic biology applications and immunotherapy.